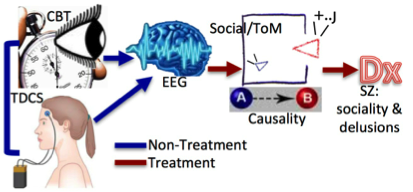
 Basic Research Questions
Basic Research Questions1. How does the perception of time influence the attributions of causality in physical and social interaction?
2. Can stimulation of the frontal cortex using Transcranial Direct Stimulation (TDCS), and subsequent cognitive behavioral training alter the perception of temporal coincidence and by extension physical causality attributions in a sustained manner?
3. What are the brain sequelae (EEG, fMRI) of such alterations?
Timing between events provides an integral gestalt grouping principle, enabling us to perceive causal interactions. Individuals with schizophrenia tend to have deficits perceiving temporal duration of stimuli. We argue that temporal windows used for identifying events as coincident or related (temporal coincidence) are less precise in individuals with schizophrenia than healthy subjects. Hyper-association of sequential events due to enlarged temporal coincidence windows could generate improper cause-and-effect attributions that predispose to delusion formation. This aberrant associative learning could cascade to faulty social comprehension and behavior in social interactions, contributing to social amotivation and avoidance. By anchoring these psychological and clinical abnormalities to basic time perception, we may be able to leverage our basic systems neuroscience knowledge to identify pathophysiologic mechanisms and thus potential avenues for intervention and remediation.
Clinical Application
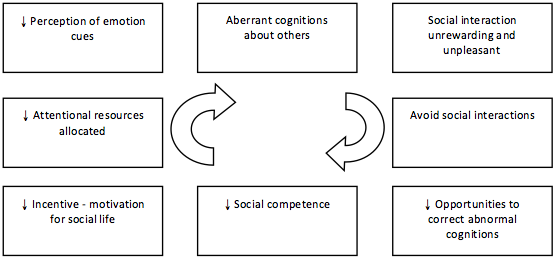 •Delusions and social communication miscues are hallmarks of schizophrenia and could both emerge from a reduced precision the temporal duration and coincidence of stimuli.
•Delusions and social communication miscues are hallmarks of schizophrenia and could both emerge from a reduced precision the temporal duration and coincidence of stimuli.
•Overly associating sequential stimuli or events could generate improper cause-and-effect groupings or attributions, the cumulative sum of which, might contribute to delusion formation.
•Similarly, excessive integration of events over time could generate aberrant associative learning resulting in faulty communication and behavior in social interactions and ultimately social amotivation and avoidance (see right).
•Anchoring such phenomena, even abstractly to elemental time perception has two advantages: 1) we can leverage our basic systems neuroscience knowledge focusing the neural mechanism of time perception interrogate these rather than complex and opaque social cognitive mechanism. 2) we can test an etiopathic model that if correct provides clear schemas for dysfunction remediation.
Behavioral Tasks
These tasks allow us to explore how distortions in perception of temporal coincidence can contribute to the perception of physical causation and social agency.
Visual Timing Task (based on task developed by M Wiener)
Simultaneity
Physical Causality (based on Woods et al. (2014) adaptation of Shalice (1964) launching experiment)
Social Agency (in development)
Techniques Used
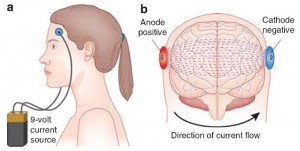
Transcranial Direct Current Stimulation (TDCS)
TDCS facilitate CBT training for time perception tasks (Vis 100 and Simultaneity). In this study, we use frontal lobe TDCS, as well as parietal lobe (sham) as a control measure. TDCS of the brain shows strong effects on behavior. While tDCS mechanisms are incompletely understood, tDCS clearly modulates oscillatory activity of large neural networks (Kuo & Nitsche, 2012). Pilot data demonstrated that tDCS over frontal cortex reduces TC window size, narrowing the period of post-impact delay under which a healthy individual will perceive launching events as causal. TDCS may increase endogenous oscillatory coherence, thereby transiently modulating temporal perception circuitry and associated judgments of causation, agency, and social interaction. Prior work in aphasia has suggested that tDCS enhances the effectiveness of subsequent cognitive-behavioral therapy (CBT) (Miniussi et al., 2008). CBT-like exercises have been shown to sharpen perception of time (meter) and pitch (Weill-Counlamountry et al., 2008). We will adapt this CBT approach, in combination with fronto-cortical tDCS, to narrow TC windows and examine if improving temporal perception can modulate judgments of causation agency and social interaction in SZ.
 Electroencephalography (EEG)
Electroencephalography (EEG)
Decades of research indicate electroencephalography (EEG) abnormalities in SZ that underpin perceptual dysfunction. High levels of poorly coherent endogenous oscillatory EEG activity are associated with reduced coordinated activity to external stimuli. For example, the muted automatic responses in SZ to auditory tones illustrate a failure to precisely mark simple temporal characteristics like stimulus onset and offset (Baldeweg et al., 2004; Lebrun et al., 2012; Leitman et al., 2010; Moreau et al., 2009; Rissling & Light, 2010). These muted responses robustly correlate with high levels of broadband incoherent power prior to stimulus onset (Lakatos et al., 2013). This suggests that reduced coherence in endogenous coherence in SZ, brain activity make them less predisposed to respond to external stimuli.

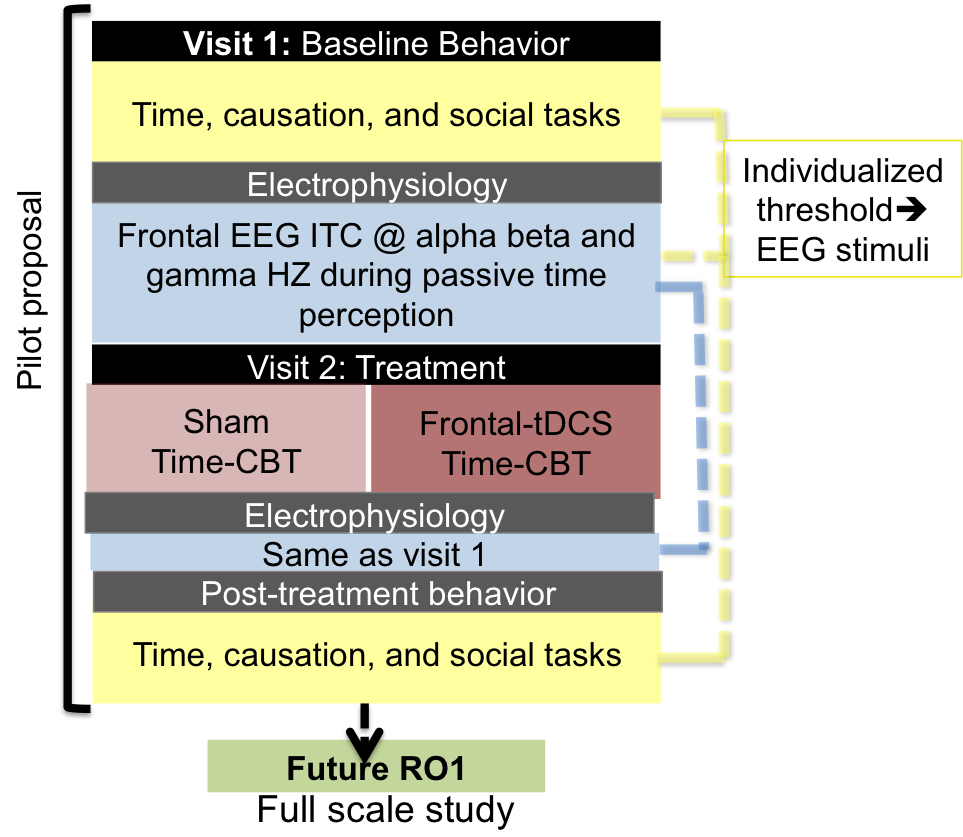
Study Protocol
Visit 1: We will conduct baseline testing of time perception (simultaneity and duration of visual events), physical causation and attribution of social intentions, together with (i) Stimulus-evoked EEG response measures of visual time perception at and twice above behavioral thresholds, and (ii) questionnaire and interview assessments of clinical symptoms and psychosocial well-being (see section 6 for a list).
Visit 2: Participants will be randomised into two groups, receiving either active transcranial Direct Current Stimulation treatment (to the prefrontal part of the brain) or sham transcranial Direct Current Stimulation (to the parietal part of the brain). In both groups, interactive Time Perception Training will follow the neurostimulation, on the simultaneity and temporal duration time perception tasks. After the transcranial Direct Current Stimulation, participants will again perform the physical causation and attribution of social intentions testing, have an EEG and complete the clinical and psychosocial measures.
References
Baldeweg, T., Klugman, A., Gruzelier, J., & Hirsch, S. R. (2004). Mismatch negativity potentials and cognitive impairment in schizophrenia. Schizophrenia Research, 69(2-3), 203–217. doi:10.1016/j.schres.2003.09.009
Kuo, M. F. & Nitsche, M. A. (2012). Effects of transcranial electrical stimulation on cognition. Clinical EEG Neuroscience, 43(3), 192-199. doi: 10.1177/1550059412444975.
Lakatos, P., Schroeder, C. E., Leitman, D. I., & Javitt, D. C. (2013). Predictive suppression of cortical excitability and its deficit in schizophrenia. Journal of Neuroscience, 33(28),11692-11702.
Lebrun, M.-A., Moreau, P., McNally-Gagnon, A., Mignault Goulet, G., & Peretz, I. (2012). Congenital amusia in childhood: a case study. Cortex, 48(6), 683–8. doi:10.1016/j.cortex.2011.02.018
Leitman, D. I., Sehatpour, P., Higgins, B. a, Foxe, J. J., Silipo, G., & Javitt, D. C. (2010). Sensory deficits and distributed hierarchical dysfunction in schizophrenia.
Miniussi, C., Cappa, S. F., Cohen, L. G., Floel, A., Fregni, F., Nitche, M. A., Oliveri, M., Pascual-Leone, A., Paulus, W., Priori, A., & Walsh, V. (2008). Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabilitation. Brain Stimulation, 1(4), 326-336. doi: 10.1016/j.brs.2008.07.002
Moreau, P., Jolicoeur, P., & Peretz, I. (2009). Automatic brain responses to pitch changes in congenital amusia. Annals Of The New York Academy Of Sciences, 1169, 191–194.
Rissling, A. J., & Light, G. A. (2010). Neurophysiological measures of sensory registration, stimulus discrimination, and selection in schizophrenia patients. Current Topics in Behavioral Neurosciences, 4, 283–309.
Weill-Chaounlamountry, A., Soyez-Gayout, L., Tessier, D., & Pradat-Diehl, P. (2008). Cognitive rehabilitation of amusia. Annales de Réadaptation et de Médecine Physique, 51(5), 332-41. doi: 10.1016/j.annrmp.2008.03.005
Woods, A. J., Hamilton, R. H., Kranjec, A., Minhaus, P., Bikson, M., Yu, J., & Chatterjee, A. (2014). Space , time , and causality in the human brain, Neuroimage, 92, 285–297
Comments are closed.